Some Important Compounds of Transition Metals
Some Important Compounds of Transition Metals: Overview
This topic covers concepts, such as, Important Compounds of d-Block Elements, Potassium Dichromate, Preparation of Iron(III) Chloride & Properties of Iron(III) Chloride etc.
Important Questions on Some Important Compounds of Transition Metals
A compound which is a strong oxidizing agent and has orange coloured crystal. It is used in the preparation of azo compounds. Identify the compound:
On oxidation of by in neutral aqueous medium, the oxidation number of S would change from:
On complete oxidation of in acidic medium, the oxidation state of Cr will change from:
The solution of potassium dichromate is prepared from which of the following compound and how its colour changes with change in pH:
The number of moles of that will be needed to react with one mole of sulphite ion in acidic solution is:
In the scheme given below, and , respectively, are
Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox Titration. Some half cell reactions and their standard potential are given below:
Identify the only incorrect statement regarding the quantitative estimation of aqueous
The following steps are involved in the manufacturing of potassium dichromate:
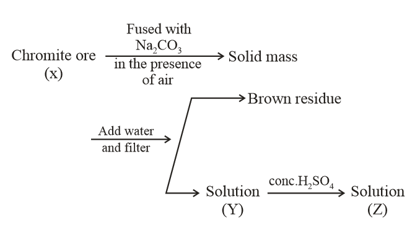
What is the difference in the oxidation number of between X and Y?
In which reaction no colour change will be observed?
Write the Physical Properties of Copper Oxide or Cupric Oxide.
Write a balanced chemical equation for the following word equation:
Dilute nitric acid on reaction with silver liberates gas.
Dilute nitric acid on reaction with silver liberates:
Silver metal reacts with hot concentrated nitric acid and produce silver nitrate salt, nitrogen dioxide gas and water as products.
What happens when silver reacts with Hot Conc. .
In the reaction , sulphuric acid acts as an acid as well as an oxidant.
In the reaction , sulphuric acid acts as :
The black coating in silver is due to the formation of silver sulphide.
Why is there a black coating in silver?
Silver articles become black on prolonged exposure to air. This is due to the formation of silver sulphide.
